The European Anti-Counterfeiting Directive and its impact on the labelling of medicines
Why introduce readers for 2D codes in healthcare now?
 The European Union's Anti-Counterfeiting Directive, which comes into force on 9 February 2019, makes it mandatory to label medicines with 2D codes. This does not apply to medicines that are for over-the-counter sale (e.g. animal charcoal, vitamins) or medicines that can be dispensed without a prescription but are at risk of counterfeiting (so-called "over-the-counter" medicines). These may not be labelled (Article 54(o)).
The European Union's Anti-Counterfeiting Directive, which comes into force on 9 February 2019, makes it mandatory to label medicines with 2D codes. This does not apply to medicines that are for over-the-counter sale (e.g. animal charcoal, vitamins) or medicines that can be dispensed without a prescription but are at risk of counterfeiting (so-called "over-the-counter" medicines). These may not be labelled (Article 54(o)).
Let us ask ourselves, why anti-counterfeiting? Not because copies of 2D codes on medicines cannot be made. The point is that every manufacturer will be obliged to register the manufactured drug in a repository. Only medicines registered in the repository will be able to be sold.
At the time of sale, the seller (pharmacy) will have to read the 2D code, see if the medicine is safe and undamaged, and invalidate it at the time of sale by reading the code. De-validation means that the sold drug is removed from the drug repository.
Are ePrescriptions related to 2D codes? Why did they start introducing 2D code readers especially for ePrescriptions?
The ePrescription is not related to the anti-counterfeiting decree. The ePrescription allows but does not mandate the use of 2D codes, it is voluntary. Pharmacies could have easily equipped themselves with a conventional 1D reader when ePrescriptions were introduced, but many pharmacies were thinking about the future and knew that they would have to take a second step a year later.
So how will this work in the new system with the anti-counterfeiting directive?
The manufacturer will be obliged to register the medicines in the central repository with other information . Depending on which market the medicine is intended for, the information from the central repository will then be transferred to the national repository. Each repository will be monitored by a certified authority. Only the manufacturer of the medicines will have the possibility to register.
At the beginning of the process, the manufacturer writes this drug information into the repository:
- The product code (GTIN)
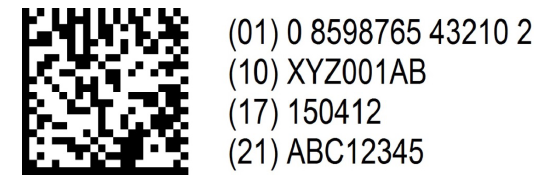
- Serial number
- Batch number
- Expiry date
- National/national reimbursement number - optional
This registration process will make the medicinal product ready for distribution. With some well-defined exceptions, the distribution will not be able to invalidate the medicine from the repository. Distribution will be passive in terms of the law with a few exceptions in the Directive. The exceptions are, for example, the sale of a medicinal product outside a specific national market or to the military.
The seller will have to invalidate the medicine at the point of sale. By reading the 2D code, he will have to invalidate the sold medicine through his information system so that it can no longer be sold or distributed. The invalidation of the medicine ends the process in the Anti-Counterfeiting Directive.
What is the significance of the anti-counterfeiting directive for the health sector, for specific pharmacies and hospitals?
The directive is important on a national scale in terms of ensuring that the drug chain in the national market does not getany drug outside the chain of the directive, any drug that is not entered into the repository by the manufacturer.
The cost of managing the repository is borne by the Czech Republic, all other costs and responsibilities remain with the manufacturers, pharmacies, hospitals.
It is unquestionably a greater responsibility, the cost of technology, the development of the information system and greater personnel costs.
Pharmacies and hospitals can only benefit from the anti-counterfeiting directive to the extent that they make maximum use of the purchased technology for their needs. It makes sense for pharmacies and hospitals to stock a given drug via a 2D code.
A 2D code reader/terminal will read all the information from the drug - GTIN, batch, serial number, expiry date - and can immediately enter it into the information system. Otherwise, these values are entered manually into the information system. It's definitely a big saving on the intake.
So, what is the recommendation for manufacturers, pharmacies, hospitals for the introduction of anti-counterfeiting guidelines?
If barcode technology is going to be fitted, then use it in such a way as to maximise the need for 2D codes. The technology should be proven, not expensive and preferably from a company that can not only service it but also set it up. There is nothing worse than having problems with the technique, or setup, in addition to the problems that the directive is sure to bring - especially in the early and transitional stages.
What equipment is suitable for meeting the anti-counterfeiting directive?
A proven device for meeting the directive is a 2D code reader. We have already successfully implemented the project in selected pharmacies. Read more HERE.
L-51X (for point of sale use)
The reader meets all the parameters for the guidelines, can read Datamatrix codes, is very cost effective, includes a stand, can be set to Czech.
The reader is suitable for use as a POS reader. This reader will invalidate the 2D code, as its readability even in case of worse printing on pharmaceuticals is excellent and proven.
Reader - 2D code terminal 8200-2D or 8231-2D (warehouse receipt)
or
The readers meet the requirements for all parameters of the directive, are very attractive in price, have excellent readability even with violatedcodes, they meet the requirements of 2D code reading - Datamatrix, they are well flexible for use in information systems, proven in practice.
Is the 2D code used in the anti-counterfeiting directive a QR code?
Manufacturers do not use QR codes for pharmaceuticals, but GS1 Datamatrix.
What is the designation of the Anti-Counterfeiting Directive and where can I find out more information?
The Anti-Counterfeiting Directive is designated 2011/62/EU.
National Organisation for the Authentication of Medicines, where you can find detailed information about the system and its implementation:
In terms of the 2D code, you can find out about standardisation and further information about the Datamatrix code for medicines at.






